Boyle’s Law: The pressure of a given quantity of gas is inversely proportional to it’s volume. Increasing volume results in decreasing pressure. Decreasing volume results in Increasing pressure. Increasing volume causes a decrease in pressure which causes air to rush in as the pressure’s reached equilibrium. Decreasing volume causes an increase in pressure which causes air to expelled as pressure’s reached equilibrium. The mechanics of breathing involve changing the volume and pressure of the thoracic cavity. By using the principles of Boil’s law, one can see that the pressure in the thoracic cavity is inversely proportional to it’s volume. When the intercostals muscles contract the ribs are elevated. At the same time the diaphragm contracts. These events expand the thoracic cavity, decreasing it’s internal pressure. The lungs expand, filling the thoracic cavity. The resulting pressure in the lungs is lower than that outside the body. Air enters the lungs until equilibrium is reached. When the diaphragm and the intercostals muscles relax the thoracic cavity recoils. The resulting increase in pressure cause the air within the lungs to be expelled.
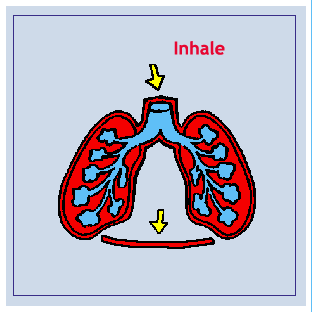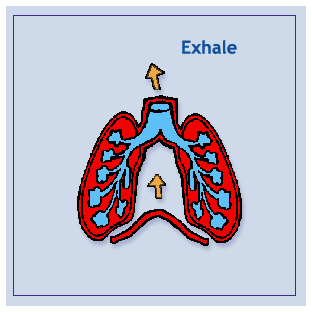
Diagram: Inhale/Exhale